Imaging of Matter
X-rays visualize how one of nature’s strongest bonds breaks
8 June 2023
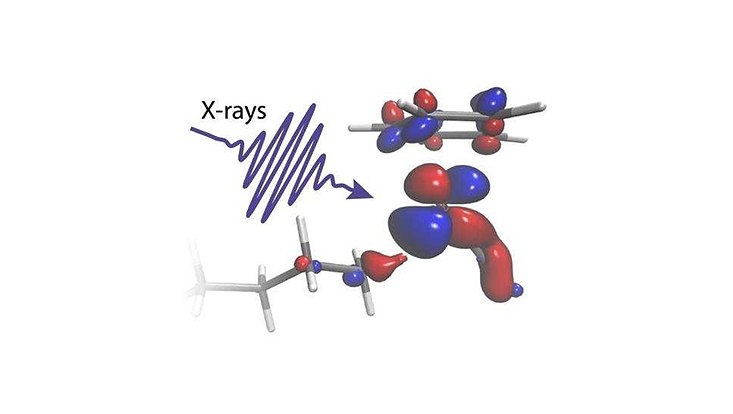
Photo: Raphael Jay
The use of short flashes of X-ray light brings scientists one big step closer toward developing better catalysts to transform the greenhouse gas methane into a less harmful chemical. The results, published in the journal Science, reveal for the first time how carbon-hydrogen bonds of alkanes break and how the catalyst works in this reaction.
Methane, one of the most potent greenhouse gases, is being released into the atmosphere at an increasing rate by livestock farming as well as the continuing unfreezing of permafrost. Transforming methane and longer-chain alkanes into less harmful and in fact useful chemicals would remove the associated threats, and in turn make a huge feedstock for the chemical industry available. However, transforming methane necessitates a first step, the breaking of a C-H bond, one of the strongest chemical bonds in nature.
Forty years ago, molecular metal catalysts were discovered that can easily split C-H bonds. The only requirement was a short flash of visible light to “switch on” the catalyst and, as by magic, break the strong C-H bonds of alkanes passing nearby without using any further energy.
“Despite the immense importance of breaking chemical bonds, the speed of such reactions on tiniest length scales below a nanometer made it impossible to watch the catalyst in a liquid break a molecule’s C-H bond. There was just no experimental way to pick out those few molecules whose C-H bonds would be broken next in a liquid of millions of constantly moving molecules”, says Nils Huse, who is a professor of physics at Universität Hamburg and a researcher in the cluster of Excellence “CUI: Advanced Imaging of Matter”.
Now, scientists were able to directly watch a catalyst at work and reveal for the first time how C-H bonds are broken. The research was led by scientists from Uppsala University in collaboration with the Paul Scherrer Institute in Switzerland, Stockholm University, Universität Hamburg within its cluster of excellence “CUI: Advanced Imaging of Matter”, and the European XFEL in Germany.
Scientist followed the delicate exchange of electrons in two experiments
In two experiments conducted at the Paul Scherrer Institute in Switzerland, the researchers were able to follow the delicate exchange of electrons between a rhodium catalyst and an octane C-H group during bond cleavage.
“The time-resolved X-ray absorption experiments we performed are only possible at large-scale facilities like SwissFEL and the Swiss Light Source, which provide extremely bright and short X-ray pulses. The catalyst is immersed in a dense octane solution, but by taking the perspective of the metal, we could specifically pick the one C-H bond out of hundreds of thousands which is made to break,” explains Raphael Jay, Researcher at Uppsala University and lead experimentalist of the study.
The reaction could thereby be followed all the way from the beginning to the end, revealing the initial light-induced activation of the catalyst within 400 femtoseconds (0.0000000000004 seconds) to the final C-H bond breaking after 14 nanoseconds (0.000000014 seconds).
Theoreticians teamed up to interpret the complex experimental data
To interpret the complex experimental data, theoreticians from Uppsala University and Stockholm University teamed up and performed advanced quantum-chemical calculations.
“Our calculations allow us to clearly identify how electronic charge flows between the metal catalyst and the C-H group in just the right proportion. We can see how charge flowing from the metal onto the C-H bond glues the two chemical groups together. Charge flowing in the opposite direction instead acts as a scissor that eventually pushes the C and the H atom apart,” explains Ambar Banerjee, Postdoctoral researcher at Uppsala University and lead theoretician of the study.
The study solves a forty-year-old mystery about how an activated catalyst can actually break strong C-H bonds by carefully exchanging fractions of electrons and without the need for huge temperatures or pressures. With their new tool at hand, the researchers next want to learn how to direct the flow of electrons to help develop better catalysts for the chemical industry in order to make something useful out of methane and other alkanes.
Reference
R. M. Jay, A. Banerjee1, T. Leitner et al.
"Tracking C-H activation with orbital resolution"
Science (2023)


