Imaging of Matter
Ultrashort optical and X-ray pulses capture elusive intermediate in catalysis
29 May 2024
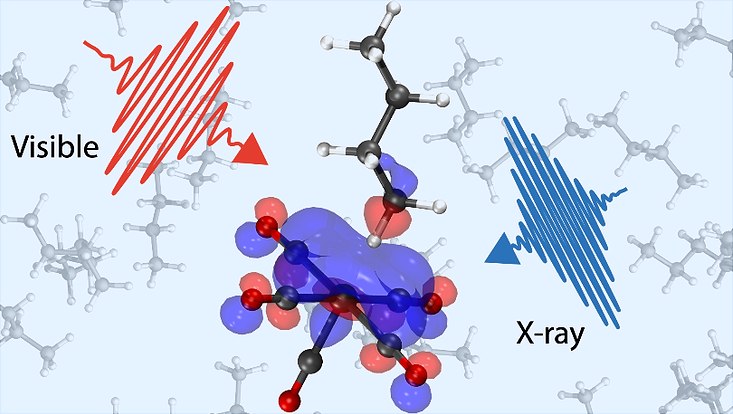
Photo: Raphael Jay
An international team of scientists has used visible and X-rays light to fully understand how the key intermediate structure forms before a carbon-hydrogen bond can be broken. The study, published in the “Journal of the American Chemical Society” (JACS), uses a combination of spectroscopic methods to resolve questions that have been debated for fifty years, the answers of which may help make better catalysts.
Transforming the greenhouse gas methane and longer-chain alkanes into less harmful chemicals constitutes one of the grand challenges in chemistry. Such transformations necessitate the breaking of carbon-hydrogen (C-H) bonds, one of nature’s strongest chemical linkages. A very attractive way of breaking C-H bonds involves metal catalysts. More than fifty years ago, it was found that illuminating such catalysts in alkane solutions with ultraviolet light results in broken C-H bonds. However, important details have remained elusive to date: What exactly happens after the catalysts are activated by light? How do the alkane molecules approach the metal catalysts? And then, how do they bind to it?
An international research team led by scientists from Uppsala University in collaboration with researchers from Universität Hamburg, Stockholm University, the Max Born Institute and the Helmholtz-Zentrum Berlin (HZB) has now succeeded in answering these questions with a combination of X-ray and visible light. It is important to understand this process in all detail because once bound to the metal, the C-H bonds can be broken with the help of the metal in so-called C-H activation reactions.
Initial activation of the catalyst within less than 100 femtoseconds
In two experiments, conducted in the laser lab of the Huse group at Universität Hamburg and at the BESSY II synchrotron at HZB, the team was able to follow how a metal catalyst made of chromium is first activated on by ultraviolet light and then binds an alkane from the surrounding solution. By using ultrashort optical pulses as well as X-ray pulses from the BESSY II synchrotron, the reaction could be followed all the way from the beginning to the end when the alkane binds to the catalytic chromium atom. The measurements revealed the initial activation of the catalyst to happen within less than 100 femtoseconds (0.0000000000001 seconds). In this first state, the catalyst is very hot, and its parts oscillate around the chromium center. Only after these oscillations cease, the alkane can approach the catalyst and bind to it in a configuration that is called a σ-complex, the formation of which takes about 8 picoseconds (0.000000000008 seconds).
“Watching the process with optical and X-ray light allowed us to get the full picture of the process. We packed the light into short pulses to take sequences of short snapshots of the reactions. The optical pulses were so short that we could capture even the fastest atomic motions on the way to the σ-complex. With X-rays, we were then able to sensitively scan in what way the bond between the metal and the alkane forms,” explains Raphael Jay, Researcher at Uppsala University and lead experimentalist of the study.
“Optical spectroscopy is one of the oldest scientific methods for following chemical reactions. Still, scientists always keep improving instrumentation, which now allows to see small details that provide the key information for understanding how the chromium complex becomes a σ-complex. But no method can tell the whole story: Different eyes see different things. That is why we used two methods with different strengths to answer long-standing questions about σ-complex formation,” adds Nils Huse, who is a professor of physics at Universität Hamburg and a researcher in the Cluster of Excellence “CUI: Advanced Imaging of Matter”. To model and interpret the complex experimental data, theoreticians from Uppsala University and Stockholm University performed advanced quantum-chemical calculations.
The study finally resolves what has been hypothesized for fifty years about how σ-complexes form and how they weaken alkane C-H bonds in C-H bond activation reactions. With their combination of methods, the researchers want to understand next how the structure of the catalyst and the specific metal element in the catalyst’s center influence the way the catalyst is activated and how it interacts with alkanes. This will allow to better direct and tailor catalysts for C-H bond activation reactions. Text: University of Uppsala, ed.
Citation
R. M. Jay, M. R. Coates, H. Zhao et al.
“Photochemical Formation and Electronic Structure of an Alkane σ-Complex from Time Resolved Optical and X-ray Absorption Spectroscopy”
J. Am. Chem. Soc. 146, 20, 14000–14011 (2024)


