Imaging of Matter
Developing future nanomedicines
15 May 2024
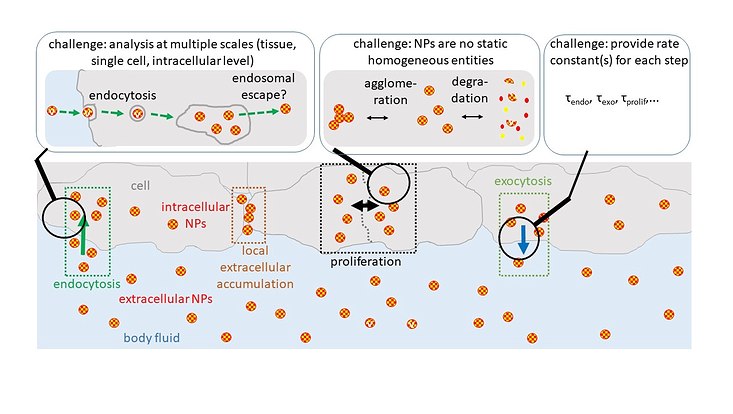
Photo: Wolfgang Parak
Quantitative analysis of biodistribution and clearance can improve the development of nanoparticles tailored to diagnostic and therapeutic needs. In a paper published in the journal "Science", Prof. Wolfgang Parak and Dr. Neus Feliu propose different imaging techniques to obtain the necessary quantitative data.
Colloidal nanoparticles (NPs) are being developed for disease treatment and diagnosis, for example, as carriers of mRNA, as agents that induce photothermal treatment of prostate cancer, or as contrast agents for magnetic resonance imaging (MRI). However, the use of NPs in clinical practice is still limited. One limitation to their use is the ability to deliver NPs specifically to their target and to control when they are effective and when they are cleared from the body.
Improved biodistribution would reduce side effects
This is related to controlling where the NPs go in the body and how local properties and dose change over time. The requirements vary depending on the application, but in general, improved biodistribution would reduce side effects. "Although most of the mechanisms of nanoparticels interaction with cells and tissues have been elucidated, quantitative details remain missing," says Prof. Wolfgang Parak from the Department of Physics at Universität Hamburg and a researcher in the Cluster of Excellence "CUI: Advanced Imaging of Matter".
Together with Dr. Neus Feliu, a researcher at the Center for Applied Nanotechnology CAN, Fraunhofer IAP, in Hamburg, Parak claims that the interaction of NPs with cells is related to the physicochemical properties of the NPs, such as size, charge and shape. As NPs can be subject to changes, compound and time-dependent changes in the physicochemical properties of NPs must be considered when biodistributions are measured. In addition, the interaction of NPs with cells does not take place in a single step.
Each pathway can be characterized by rate constants
In principle, each pathway can be characterized by one or more rate constants, the scientists say. Knowing these rate constants and how they depend on the physicochemical properties of NPs could make it possible to predict the fate of different types of NPs from their entry into the body until their possible excretion in urine or stool. Consequently, the physicochemical properties of NPs could be tailored to best suit the delivery method, target site, and time to excretion.
Therefore, datasets of the rate constants for all the pathways involved at the tissue, cellular, and intracellular levels according to the physicochemical properties of NPs could inform the design of NPs so that they have appropriate properties for optimized delivery and excretion.
Parak and Feliu propose several different techniques such as electron and optical microscopy or mass spectrometry to determine such datasets. X-ray fluorescence imaging (XFI) could be an option in the future. The major hurdle still to overcome is the potential for radiation damage during imaging. In addition, like optical near-infrared imaging in tissue, XFI also suffers from scattering effects. However, the background can be subtracted through non-isotropic scattering, paving the way for future imaging deep inside tissue.
Citation
Neus Feliu and Wolfgang J. Parak
"Developing future nanomedicines”
Science, 384, 6694, pp. 385-386 (2024)