Imaging of Matter
Enhancing photocatalysis with supercrystals
5 December 2023
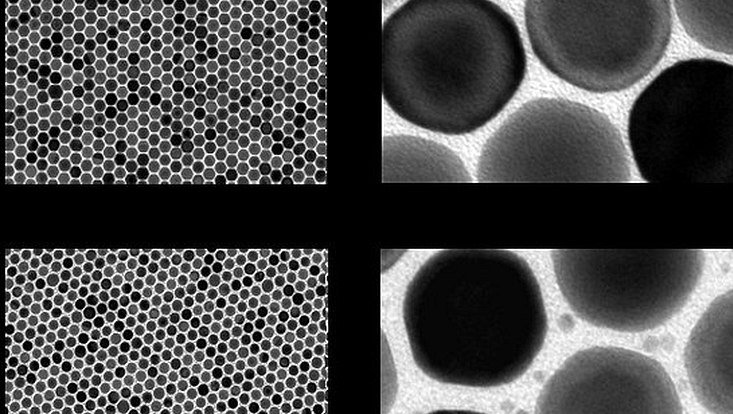
Photo: UHH, Florian Schulz
Hydrogen is the hope for a climate-friendly future. Currently, it is mostly produced by electrolysis of water with electricity, but there are other strategies, such as photocatalysis. Researchers at Universität Hamburg, Freie Universität Berlin and Ludwig-Maximilian-Universität München report in the journal Nature Catalysis how supercrystals of gold nanoparticles can turn catalytic platinum particles into photocatalysts.
In photocatalysis, light activates a catalytic chemical reaction. Photocatalytic hydrogen production with sunlight is an important process for energy production. A wide range of materials for hydrogen catalysis is known from fundamental research on heterogeneous catalysis. But most of them, such as platinum or palladium, cannot be activated by visible light. The energy needed to drive the reaction must be provided externally in a different way, for example electrochemically.
In recent years, researchers at Universität Hamburg have developed strategies for using these metals with light despite this lack. They produced so-called supercrystals from gold nanoparticles in the form of periodically arranged films and studied them. With this set-up, they were able to demonstrate an extremely strong coupling between light and the supercrystal, causing the light to condense into the spaces around the nanoparticles. Follow-up studies showed that these spaces can be infiltrated with smaller particles, such as of the catalytically highly active materials platinum or palladium. In the current collaborative project led by the LMU Munich catalysis group of Prof. Dr. Emiliano Cortés, the researchers then investigated the extent to which the properties of the catalysts change.
When excited with visible light, Platinum is initially not photocatalytically active, meaning that the reaction-enhancing properties of the material remain the same when irradiated with visible light. "However, as soon as the platinum is embedded in a gold nanoparticle supercrystal, it can be activated optically, namely in the wavelength range in which the supercrystal condenses light most strongly: in the visible, green spectral range," explains Prof. Holger Lange from the Department of Chemistry at Universität Hamburg, who also conducts research in the Cluster of Excellence "CUI: Advanced Imaging of Matter." The "passive catalyst" thus becomes a photocatalyst.
Subsequently, the scientists were able to show that other metal particles besides platinum can be embedded in the supercrystals and activated in the same way. "By designing the supercrystals and choosing the metal particles, flexible and specific photocatalysts could thus be designed," says Dr. Florian Schulz from the group of Prof. Wolfgang Parak from the Institute for Nanostructure and Solid State Physics at Universität Hamburg. To enable applications in the field of hydrogen production, the next steps are optimizing the scalability of the syntheses and the stability of the structures.
Citation
Matias Herran, Sabrina Juergensen, Moritz Kessens et al.
"Plasmonic bimetallic two-dimensional supercrystals for H2 generation"
Nature Catalysis (2023)