Imaging of Matter
Another step in the fight against Alzheimer's disease
23 March 2023
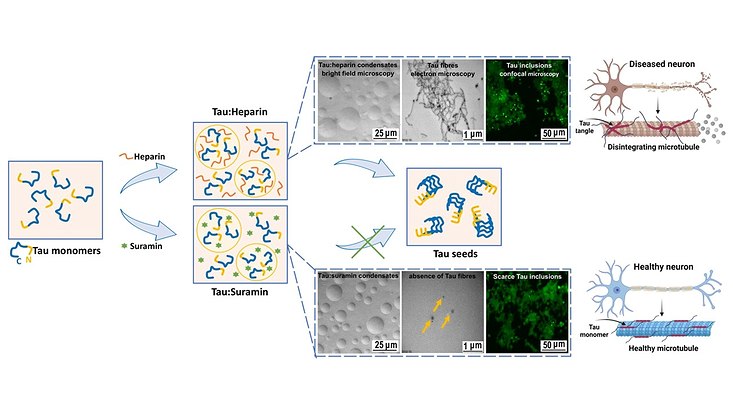
Photo: AG Betzel
The course of Alzheimer's dementia can be positively influenced by various drugs - but the brain disease cannot yet be stopped or cured. In the research journal Scientific Reports, a team led by Prof. Christian Betzel of Universität Hamburg outlines a possible therapeutic approach.
Alzheimer's dementia is one of the so-called Tauopathies: defective Tau proteins are deposited on the nerve cells and restrict them to the point of incapacity. Tau is an intrinsically disordered neuronal protein that, when aggregated, becomes the main component of the neurofibrillary tangles observed in Alzheimer's disease.
"We wanted to find out how exactly the aggregation of the Tau protein is triggered in order to possibly be able to specifically counteract it," says Christian Betzel, who also conducts research in the Cluster of Excellence "CUI: Advanced Imaging of Matter." In the experiment, Tau aggregation can be triggered by polyanionic cofactors such as RNA or heparin. The same polyanions at different concentration ratios generate Tau condensates via liquid-liquid phase separation (LLPS), which develop pathological aggregation potential over time.
The scientists performed time-resolved dynamic light scattering experiments (trDLS) and small-angle X-ray scattering experiments at DESYs light source PETRA III in combination with electron microscopy investigations at the Biolaboratory (XBI) of the European Free Electron Laser EuXFEL to analyze the process in detail with high spatial and temporal resolution.
Electrostatic interactions minimize the potential to form tangles
They found that electrostatic interactions between Tau and the negatively charged drug suramin lead to the condensation of Tau. These interactions compete with the interactions that drive the formation of Tau:heparin and Tau:RNA coacervates. As a result, the potential to trigger cellular Tau aggregation decreased. Even after extended incubation, Tau:suramin condensates did not trigger Tau aggregation.
“Our results provide evidence that the dynamic exchange between polyanionic polymers like heparin and RNA and anionic molecules like suramin can modulate the phase separation of Tau,” says Prabhu Rajaiah Prince, Postdoc at the Cluster of Excellence. Further modifications offer the possibility of tuning the function of Tau depending on the local cellular environment. This information could help in the search for modulators of Tau condensation, the researchers say, that disassemble Tau coacervates or prevent their transition into Tau seeds. The goal would be to develop therapeutics that prevent Tau aggregation in the brain.
The researchers also propose an analogy to the theory of colloidal suspension stability: Minimal interactions between colloidal Tau particles could trigger Tau aggregation. Prince: "We believe that trDLS is an ideal analytical tool to analyze the effect of polyanionic compounds on protein condensation and is well suited to investigate these processes on a molecular level."
Researchers from the German Center for Neurodegenerative Diseases (DZNE) in Bonn and Berlin, the University of Bonn and the European XFEL were also involved in the work.
Citation
P. R. Prince, J. Hochmair, H. Brognaro, S. Gevorgyan, M. Franck, R. Schubert, K. Lorenzen, S. Yazici, E. Mandelkow, S. Wegmann & Ch. Betzel
"Initiation and modulation of Tau protein phase separation by the drug suramin“
Scientific Reports 13, 3963 (2023)