Imaging of Matter
How far can a proton make its presence felt when embedded in water?
1 December 2022
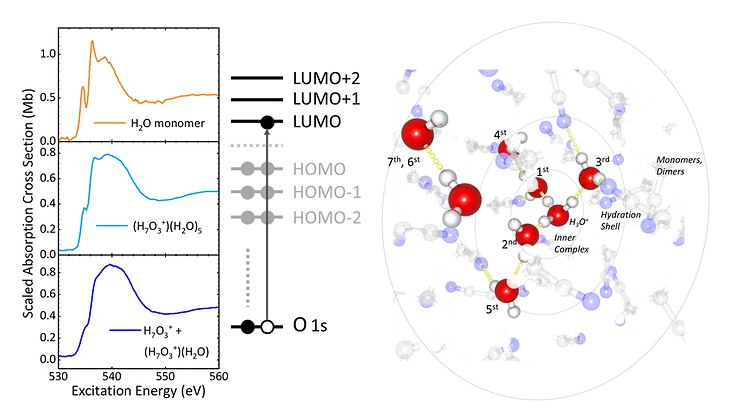
Photo: Erik T. J. Nibbering
An international team of researchers has gained important insights into the electronic structure of hydrated proton complexes in solution: The involvement of a certain number of water molecules in the uptake of an excess proton in aqueous environments exhibits a structural hierarchy that is more extensive than is often assumed. This new finding could be important in understanding proton transport in energy conversion in fuel cells or signal transduction in transmembrane proteins.
The work involved research teams from the Max Born Institute, Universität Hamburg and the Cluster of Excellence "CUI: Advanced Imaging of Matter", Stockholm University, Ben Gurion University of the Negev and Uppsala University.
Read the full press release from the Max Born Institute.
Original Publication
M. Ekimova, C. Kleine, J. Ludwig, M. Ochmann, T. E. G. Agrenius, E. Kozari, D. Pines, E. Pines, N. Huse, Ph. Wernet, M. Odelius and E. T. J. Nibbering
"From Local Covalent Bonding to Extended Electric Field Interactions in Proton Hydration"
Angewandte Chemie International Edition 61, e202211066 (2022)