Imaging of Matter
Fighting antibiotic resistance: DESY scientists advance drug development with room-temperature X‐ray screening
16 October 2025

Photo: DESY
CUI member Prof. Henry Chapman and researchers at the Center for Free-Electron Laser Science CFEL at DESY have developed a new room-temperature X‐ray crystallography technique that could significantly accelerate structure-based drug discovery—a process where drug candidates are tailored using the 3D structure of proteins.
This approach shows proteins in states closer to their natural, physiological conditions. The study, published in nature communications, demonstrates that this method produces protein structure data comparable to conventional cryogenic experiments in quality, while also revealing previously unknown spatial variations–so-called “conformations”–of medically relevant proteins. In fact, during testing at room-temperature, the team discovered a previously unseen conformation of an enzyme responsible for unwanted antibiotic resistance. This key finding opens up new avenues for the development of drugs that prevent antibiotic resistance.
Protein structure analysis at room temperature
Macromolecular X-ray crystallography has long been a cornerstone of drug development, enabling scientists to visualise interactions between proteins and drug candidates with atomic resolution. Traditionally, these experiments are performed at cryogenic temperatures (about –170 °C) to minimise radiation damage to samples and prevent dehydration. However, freezing can distort molecular structures and mask dynamic states crucial for biological function and drug efficacy.
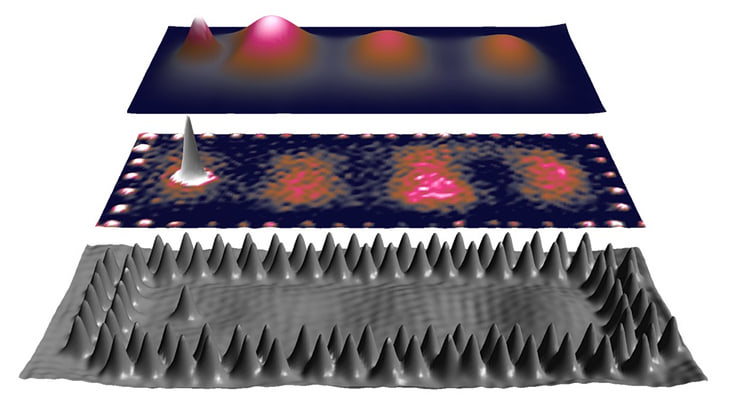
The DESY team has now shown that serial crystallography at room temperature can overcome these limitations. Using the newly developed HiPhaX (High-throughput Pharmaceutical X-ray screening) instrument at the PETRA III X-ray source, the researchers systematically compared fragment screening at 23 °C, i.e. much closer to physiological conditions, with classic measurements at –170 °C for an enzyme involved in bacterial antibiotic resistance. Fragment screening is a simplified drug screening approach using small molecules, fragments of potential drugs, to rapidly reveal basic interactions with proteins, providing blueprints for further drug development steps.
“Room-temperature crystallography allows us to observe proteins in a state much closer to their natural function in living organisms,” says DESY researcher Sebastian Günther, lead author of the study. “By combining serial crystallography with on-chip crystallisation and advanced multi-sample holders, we can rapidly and reliably screen compounds under nearly physiological conditions.”
HiPhaX: a new Instrument for pharmaceutical X-ray screening
Purpose-built for high-throughput serial crystallography, the new HiPhaX instrument enables rapid data collection from thousands of microcrystals, precisely controlling both temperature and humidity. With its sample holders equipped with 12 compartments and integrated automation, HiPhaX makes it possible for researchers to efficiently screen large numbers of potential protein-drug interactions with minimal manual effort and very little sample material.
Discovering a hidden protein conformation
With this method, the team uncovered a previously unobserved conformation in the active centre of the enzyme that affects its interactions with antibiotics and potential drug molecules. This conformational state was invisible in previous cryo-crystallography datasets and even missing from the predictions of AlphaFold 3, the most advanced AI tool for protein structure prediction to date. This discovery underlines the value of experimental data collected at physiological temperatures, highlighting functionally relevant protein states that computational models can overlook.
“HiPhaX represents a major advance for structural biology,” says Alke Meents, lead author of the study. “It combines speed, precision, and flexibility. The fact that AlphaFold did not predict this conformation shows the enormous potential for synergy between experiment and AI. By providing realistic room-temperature data, we can help train future computational models to be more accurate and biologically relevant.”
Looking ahead: faster discoveries at PETRA IV
The new method developed by the team could reach its full potential at PETRA IV, the planned successor to the current PETRA III synchrotron at DESY. “More extensive sample screenings open up new channels from fundamental research to drug development. With PETRA IV, these processes will be even faster and more efficient in future thanks to extensive automation and the use of artificial intelligence,” says Britta Redlich, Director of Photon Science at DESY.
PETRA IV will provide X-rays of unprecedented brilliance, enabling ultrafast, fully automated structural screenings and further boosting the infrastructure for drug discovery.
Towards faster, more realistic drug development
The study suggests that room-temperature fragment screening could soon become standard in structure-based drug design. By combining this highly automated X-ray screening with AI-powered data analysis, thousands of compounds could be analysed each day in the near future. This will substantially accelerate the journey from molecular discovery to medical application.
The work was carried out at DESY’s PETRA III synchrotron and supported by the Helmholtz Association and the German Federal Ministry of Education and Research (BMBF, now BMFTR) as part of the Röntgen-Ångström-Cluster project “X-ray drug design platform (XDD).” Text: DESY, ed.
Citation
Günther, S., Fischer, P., Galchenkova, M. et al.
Room-temperature X-ray fragment screening with serial crystallography
Nat Commun 16, 9089 (2025).