Imaging of Matter
ERC Synergy Grant: circa €11 million for the “GRAIL“ Research ProjectNew instrument for the study of life
26 October 2023
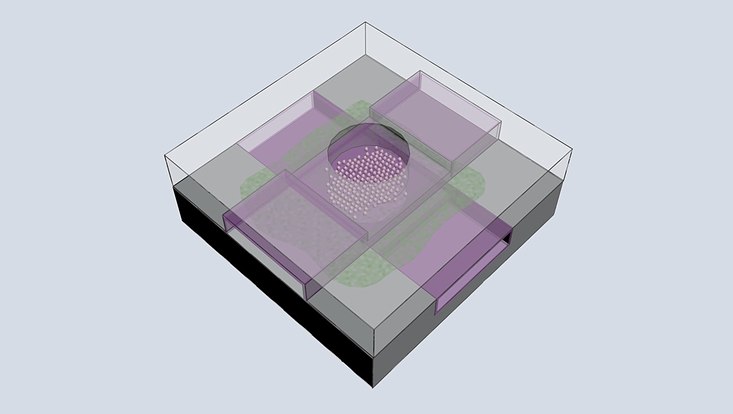
Photo: Pearson, Fernandez-Cuesta
All living systems are permanently in motion. But what exactly happens when proteins are at work? Researchers from Universität Hamburg and the University of Groningen have been awarded a Synergy Grant from the European Research Council for their novel approach to studying the structure-function dynamics of membrane proteins. Their project "GRAIL" will be funded with circa €11 million for six years.
The lynchpin of the new research project are gradients, i.e. the permanently changing chemical conditions inside and outside the lipid membranes that surround cells, and how these gradients affect the structure and function of the integral membrane proteins embedded within the membranes. Since their discovery in the 1960s, the biomedical importance of chemiosmotic gradients for membrane protein function has become increasingly clear. Today, integral membrane proteins are the major targets for drug development, such as for the treatment of Parkinson's disease and cancer, or to overcome antibiotic resistance.
"Despite their importance, it has to-date not been possible to directly observe the structural dynamics of proteins in biological membranes in the presence of these physiologically vital gradients," says Prof. Arwen Pearson of the Institute of Nanostructure and Solid State Physics at Universität Hamburg, who specializes in time-resolved structural biology and is coordinating the project. “In the GRAIL project, we aim to close this fundamental gap in our understanding of some of the most basic processes of life by developing a new method to watch membrane proteins in action and in the presence of a chemiosmotic gradient”. GRAIL builds on research conducted in the Cluster of Excellence "CUI: Advanced Imaging of Matter", the Marie-Curie Sklodowska International Training Network “Neurotrans”, and the ERC Starting Grant of Team member Fernandez-Cuesta, “Fluinems”. All of these have provided the key preliminary result to make the new project possible. The team have been further supported by the strategic partnership between Universität Hamburg and the University of Groningen.
Combined expertise in membrane proteins, photochemistry and nanotechnology
Together with Groningen researchers Prof. Dirk Slotboom of the Institute of Molecular Biosciences and Biotechnology and Dr. Wiktor Szymanski, professor of medicinal chemistry, photopharmacy and imaging, and Dr. Irene Fernandez-Cuesta, young investigator in CUI and an expert in nano-opto-fluidics, the GRAIL team combines the necessary expertise in membrane proteins, photochemistry and nanotechnology to make their vision a reality. Using a partitioned liquid cell nanodevice specially designed and fabricated by Fernandez-Cuesta, the team will focus on four carefully selected proteins from the Slotboom lab that transport protons, inorganic anions, and organic ions across the membrane. So-called photocages developed by Szymanski will allow the activity of the membrane proteins to be switched on on demand, so that Pearson can use time-resolved serial crystallography to record a movie of the membrane protein in action.
“The data GRAIL will deliver will give us totally new insights into fundamental membrane protein biophysics, as well as the deeper mechanistic understanding needed to understand better, for example, why mutations in membrane transporters can cause severe diseases of the mind, or how drugs of abuse act at the molecular level,” Pearson says. In the longer term the team hopes that GRAIL’s methodology will become as much part of the structural biology toolkit as normal crystallography, cryoEM and NMR are today.


