Imaging of Matter
Towards a non-carbon future
28 January 2020
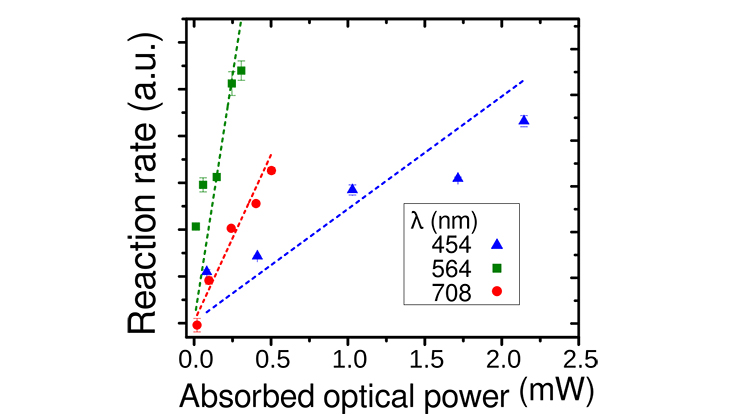
Photo: Lange group
A group of scientists led by Holger Lange from the Universität Hamburg has analyzed the different effects underlying plasmon-enhanced catalysis. The researchers observed that non-thermal effects contribute significantly to the plasmonic enhancement, which is relevant for a wide range of reactions, for example water splitting. The results have just been published in ACS Catalysis.
“Catalysis on optically excited metal nanoparticles is presently intensively discussed as a feasible approach for light-driven chemical conversions”, explains Lange, who is a member of the cluster of excellence “CUI: Advanced Imaging of matter”. Metal nanoparticles, such as gold, possess a unique optical excitation, the plasmons. There, the mobile electrons within the nanoparticle oscillate in phase with the external light. After the optical excitation, the plasmon decay leads to a non-equilibrium (non-thermal) distribution of energetic electrons. Electronic transitions and electron-electron scattering then result in an equalization of the excess energy, cooling them to thermal equilibrium. Subsequent interaction with the gold lattice ultimately turns the absorbed energy into heat. In principle, both, the non-thermal carriers as well as the elevated temperatures, can couple with a chemical reaction occurring at the nanoparticle surface. “The distinguishing between thermal and non-thermal contributions is presently a matter of debate and necessary for optimizing the plasmonic platforms,” Lange says.
Glucose oxidation - a reaction of broad relevance
To investigate the plasmon-based reaction enhancement, the scientists teamed up with electrochemistry experts at the Hamburg University of Technology (Eich group) and monitored glucose oxidation as a model reaction of broad relevance and with a reaction rate that can be directly deduced from the electrochemical current. As they expected different dependencies for thermal and non-thermal processes, they monitored this current (reaction rate) for excitations with different wave lengths.
“We observed that the reaction rate is maximized when exciting the plasmon,” explains lead author Marina Rodio, who is a Louise Johnson Fellow at the cluster. “Upon normalization on the absorbed energy, we see clear indications that non-thermal electrons drive the reaction. The same amount of energy absorbed at the plasmon resonance leads to more reaction events than that amount of energy absorbed in different spectral regions.”
Optimizing plasmonic platforms
The results are of immediate relevance for plasmon-assisted catalysis, as knowledge of the enhancing process enables optimizing the plasmonic platforms. “Our results can be tested for a wide range of reactions, relevant for a non-carbon future”, Lange says. For example, first experiments indicate that the addition of plasmonic nanoparticles to photoelectrical cells can enhance the rate of water splitting during illumination with visible light. Text: CUI
Publication:
Marina Rodio, Matthias Graf, Florian Schulz, Niclas Sven Mueller, Manfred Eich, Holger Lange
“Experimental Evidence for Non-Thermal Contributions to Plasmon-Enhanced Electrochemical Oxidation Reactions”
ACS Catal. 2020, XXXX, XXX, XXX-XXX